In this brief we explore the introduction and implications of the MEB-ZIN Parallel Procedure pilot, which was launched in 2019 in the Netherlands. Experts Eline Middelburg, Cédric Driessen, Alexander Vos, and Anke Ritte cover the rationale for its creation, as well as an overview of products that have gone through the process so far.
First results of the MEB-ZIN Parallel Procedure pilot showcases acceleration of patient access through enhanced collaboration
On average, the period between EU market approval and patient access in the Netherlands can span several months, which can be detrimental to patient access to potentially life-saving medicines.
In an attempt to shorten this timeline, the Medicines Evaluation Board (MEB) and the Care Institute Netherlands (ZIN), two key bodies involved in the Dutch market access procedure, launched a pilot which introduces a redesigned parallel flow.
This flow condenses two phases to shorten the market access period in the Netherlands:
- The market authorization phase –performed by the European Commission (EC) and MEB as part of the European Medicines Agency (EMA)
- The reimbursement approval phase (controlled by ZIN)
This “MEB-ZIN Parallel Procedure pilot” was launched in April 2019 and has already accelerated market access for three innovative medicines.
Streamlining of different registration and reimbursement phases can potentially reduce the time to patient access by 90 days
Making innovative drugs accessible to patients as soon as possible is a priority for patient organizations, health care practitioners (HCPs), the pharmaceutical industry, as well as the Dutch government.
Making innovative drugs accessible to patients as soon as possible is a priority for patient organizations, health care practitioners (HCPs), the pharmaceutical industry, as well as the Dutch government. A 2019 study uncovered that for “Sluis” products the delay between EMA approval and Dutch market access could be up to eight months to one year, accumulated throughout the different phases of the approval process. In 2015, ZIN was advised to "remove duplicate administrative procedures from the authorization and reimbursement phase". Ultimately, the MEB and ZIN decided to collaborate and create the “Parallel Procedure” to remedy this. |
MEB and ZIN start the procedure 180 days after EMA registration is filed
The revised procedure (see image below) accelerates the start of the ZIN procedure, i.e. at day 180 in the EMA approval procedure. Although most information required for an assessment is present at this stage, the modified process requires closer collaboration between the pharmaceutical company and ZIN, as the procedure begins before the approval is finalized at EMA level.
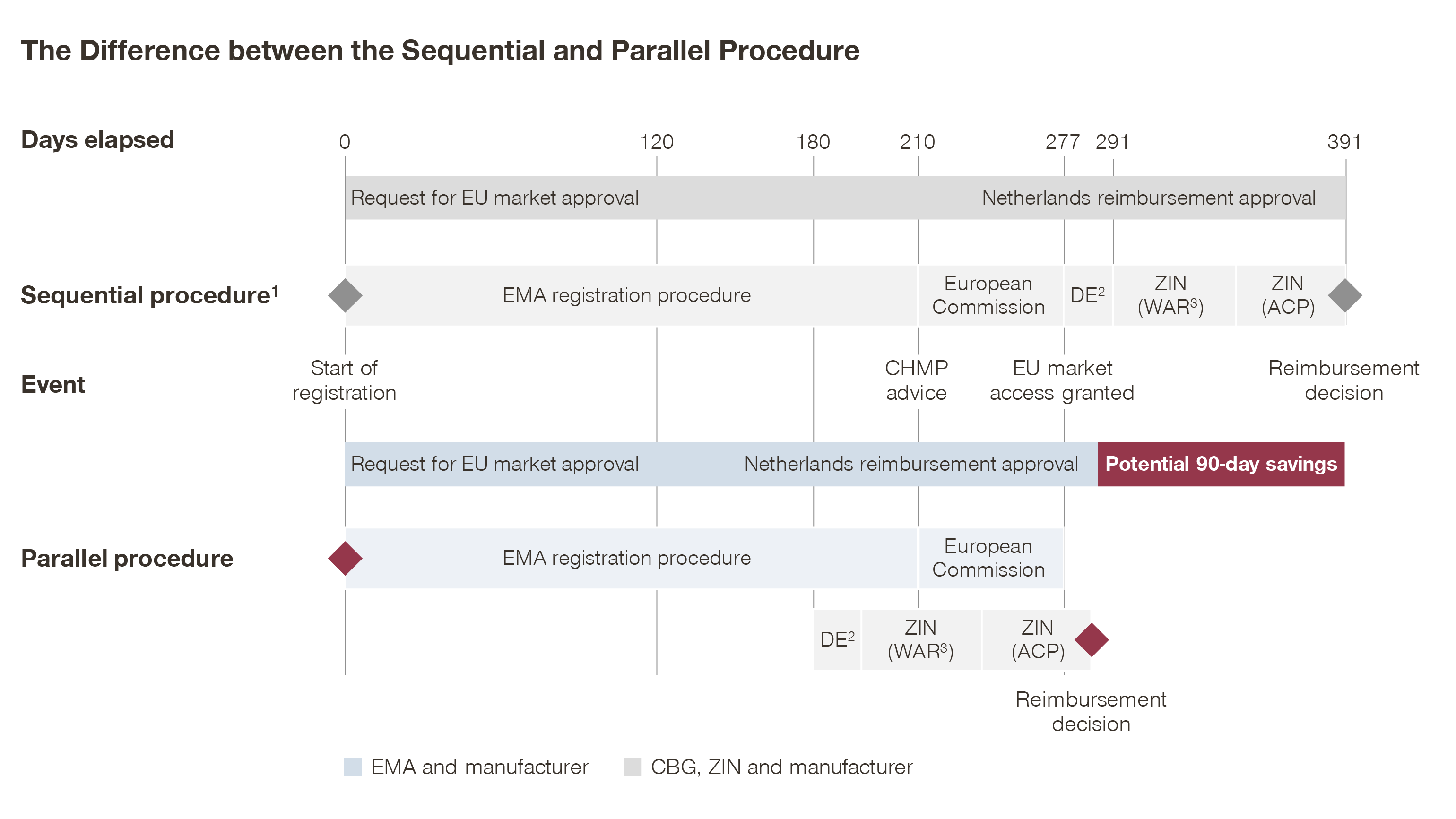
The revised procedure enables companies to start the ZIN procedure 30 days earlier (at day 180 instead of day 210), as ZIN concluded that at this moment most documents required for reimbursement approval are submitted to EMA. Aside from this, the phases remain unchanged.
Due to the earlier start, some documents might require additional clarification, making clear communication between pharmaceutical companies, MEB and ZIN a key to success. The additional communication touchpoints are agreed upon before the start of the Parallel Procedure process. Final reimbursement approval is given after marketing authorization approval by the European Commission, which avoids situations where MEB–ZIN would grant reimbursement without EU market access.
Seven innovative drugs have so far been included in the pilot
To ensure smooth organization of the pilot and that drugs accepted for the pilot are innovative, MEB and ZIN have defined several criteria:
- The marketing authorization application is classified as a ‘regular’ centralized marketing authorization application (not a major type II variation – extension of the indication)
- The registration procedure for this application and day 120 of the registration start or occur within a period predefined by MEB-ZIN
- Placement of the product has been requested on ‘List 1B’ of the retail reimbursement system (GVS) or it is with great certainty that the product will be placed in the so-called ‘Sluis’
- To help shape the Parallel Procedure, the participating pharmaceutical company is expected to cooperate proactively during the pilot (including consultation meetings, one-on-one evaluation moments)
If these criteria apply to the drug in question, the manufacturer can apply through an online form to be included in the Parallel Procedure.
Until now, seven pharmaceutical companies have been accepted in the pilot and three drugs have obtained market access:
- Rybelsus (an oral treatment for Type II Diabetes)
- Arikayce (an aerosol to treat Mycobacterium Avium Complex)
- Evrenzo (used to treat anemia)
The four other innovative drugs currently being under review are:
- A CAR-T therapy targeting multiple myeloma
- Lenacapavir, which is targeted towards multi-drug resistant HIV-1
- A drug treating diabetes type II associated with kidney failure
- A drug for the treatment of anti-neutrophil cytoplasmic autoantibody-associated vasculitis
It is also of note that Rybelsus had one of their first EU-wide launches in the Netherlands via this procedure.
First results indicate the Parallel Procedure pilot has been successful, reducing market access time on average by two and a half months
In the case of the approved drugs, the time between EMA approval and reimbursement approval by ZIN was 16 days on average, with a minimum of nine days and a maximum of 28 days. This represents a decrease in time of two and a half months, compared to the findings of previous reports showing that the Parallel Procedure could accelerate market access in the Netherlands.
Transformation towards a standard procedure is expected
The MEB-ZIN pilot has proven to be popular amongst pharmaceutical companies trying to accelerate their market access in the Netherlands for high-cost pharmaceuticals. Application records show that both large and smaller pharmaceutical companies found their way to the redesigned procedure.
Although not all applications met the requirements set by ZIN, the first iteration of the Parallel Procedure was such a success that the MEB-ZIN pilot is currently still accepting registrations (first iteration to register was initially planned between August 2019 and April 2020, but has been extended beyond this timeframe).
Additionally, there are strong signs that the current MEB-ZIN pilot will be transformed into a standard procedure in the future, albeit with modifications based on additional learnings from the pilot.
As the pilot matures in the Netherlands, market access institutions from other EU countries have expressed interest in the procedure and its findings. This interest could indicate that other countries are looking to improve patient access as well, and that this type of procedure might become more commonplace in the EU in the future.