Minimal residual disease (MRD) is redefining oncology diagnostics. This article covers the current landscape, key barriers, and emerging opportunities, and outlines what it will take to drive successful adoption at scale.
The modern oncology continuum envisions a seamless flow from prevention and early detection through treatment selection, response monitoring, and recurrence surveillance. Historically, this continuum was siloed: diagnosis by imaging or tissue biopsy, treatment by standardized protocols, and follow-up via periodic scans.
Today, new tools are converging to enable precision-based, dynamic decision-making at each juncture:
- Multi-Cancer Early Detection (MCED): Blood-based assays that screen asymptomatic individuals for cancer signals across multiple tissue types, aiming to catch malignancy well before clinical symptoms emerge
- Minimal/Molecular/Measurable Residual Disease (MRD): MRD testing now plays a central role in post-surgery surveillance, detecting trace levels of disease when conventional imaging or pathology shows no visible tumor
- Comprehensive Genomic Profiling (CGP): Deep sequencing of tumor tissue or circulating tumor DNA (ctDNA) to identify actionable mutations for targeted therapies
- Tumor Response Monitoring (TRM): Quantitative assessment of tumor burden and dynamics using imaging metrics and circulating biomarkers
By integrating CGP-informed treatment, TRM feedback loops, and MCED/liquid biopsy screening, MRD bridges key gaps in recurrence surveillance and enables earlier, more targeted interventions. It stands out as a future-defining bet for the diagnostics industry in addressing recurrence prevention and long-term oncology management.
What is MRD? |
| Minimal residual disease refers to the small number of cancer cells that survive initial therapy and remain in the patient’s body, often undetectable by standard imaging or histopathology. In solid tumors, MRD is measured indirectly - typically via ctDNA assays that detect tumor-specific genetic or epigenetic signatures in blood, saliva, or other biofluids with high sensitivity (down to variant allele frequencies of 0.01% or lower). |
In traditional practice, patients undergo definitive therapy (surgery, radiotherapy, systemic treatment) followed by scheduled imaging at defined intervals (e.g., CT scans every three to six months) and clinical assessments. Relapse is often detected when disease reaches radiographic thresholds, by which time progression may already be significant.
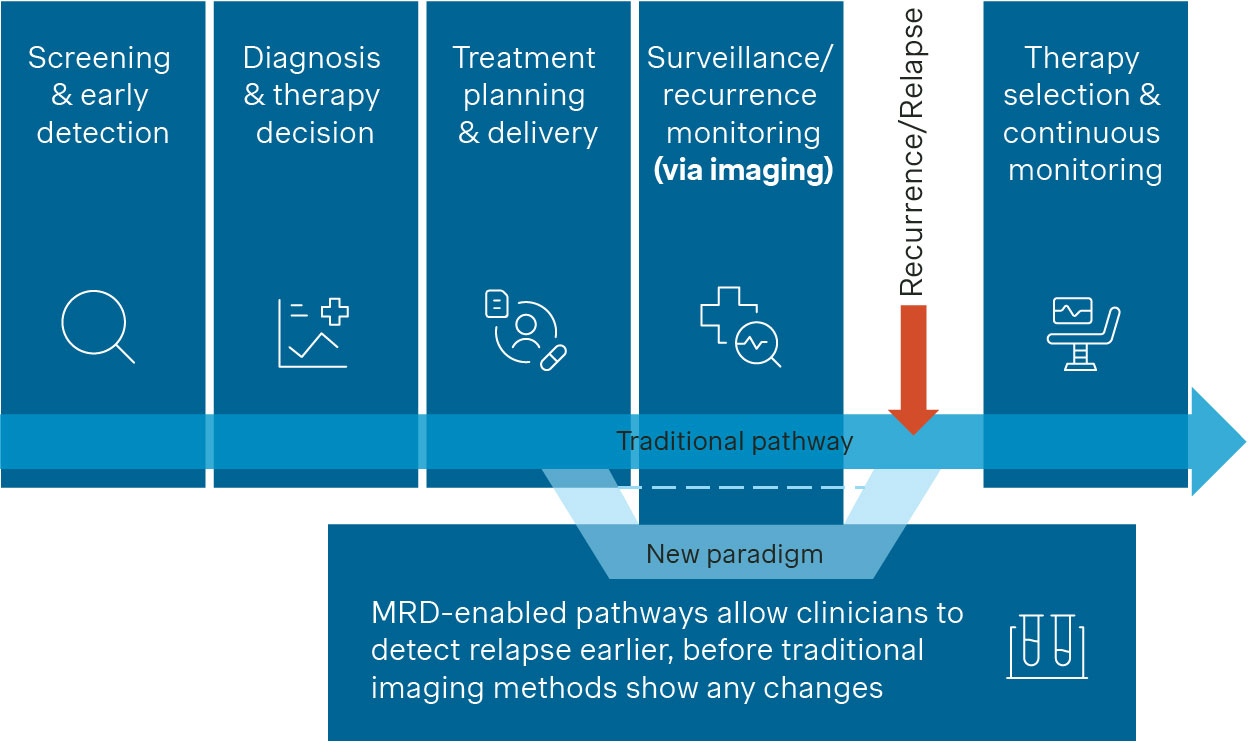
Figure 1. Traditional cancer care pathway and MRD’s impact
MRD-enabled pathways (Figure 1) allow clinicians to detect molecular relapse early. Sampling blood or other fluids after (neo)adjuvant treatment can reveal residual disease long before imaging shows changes. A positive MRD status could prompt therapy escalation such as introducing intensified chemotherapy or adding immunotherapy / targeted agents). It may also warrant closer monitoring given the strong association with recurrence. On the other hand, sustained MRD negativity may support therapy de-escalation e.g., by taking the patient off treatment, reducing toxicity and cost.
Expanding a success case in hematology
In hematologic malignancies such as acute lymphoblastic leukemia or chronic lymphocytic leukemia, MRD testing using flow cytometry or PCR is already well established as standard-of-care. Studies have demonstrated that MRD status following induction or consolidation therapy is a strong predictor of relapse risk and overall survival, guiding decisions around treatment intensification or de-escalation. These successes have driven growing interest in translating MRD to solid tumors.
While challenges such as tumor heterogeneity and lower ctDNA shedding complicate this transition, ongoing technological advances are steadily improving the sensitivity, reliability, and clinical utility of MRD testing in solid oncology. The ambition is clear: to replicate, if not fully mirror, the role MRD plays in hematology and move toward becoming a routine component of cancer care.
Potential clinical benefits for MRD testing in solid tumors
MRD testing in solid tumors can result in multiple clinical and health benefits for the oncology patient (Figure 2).
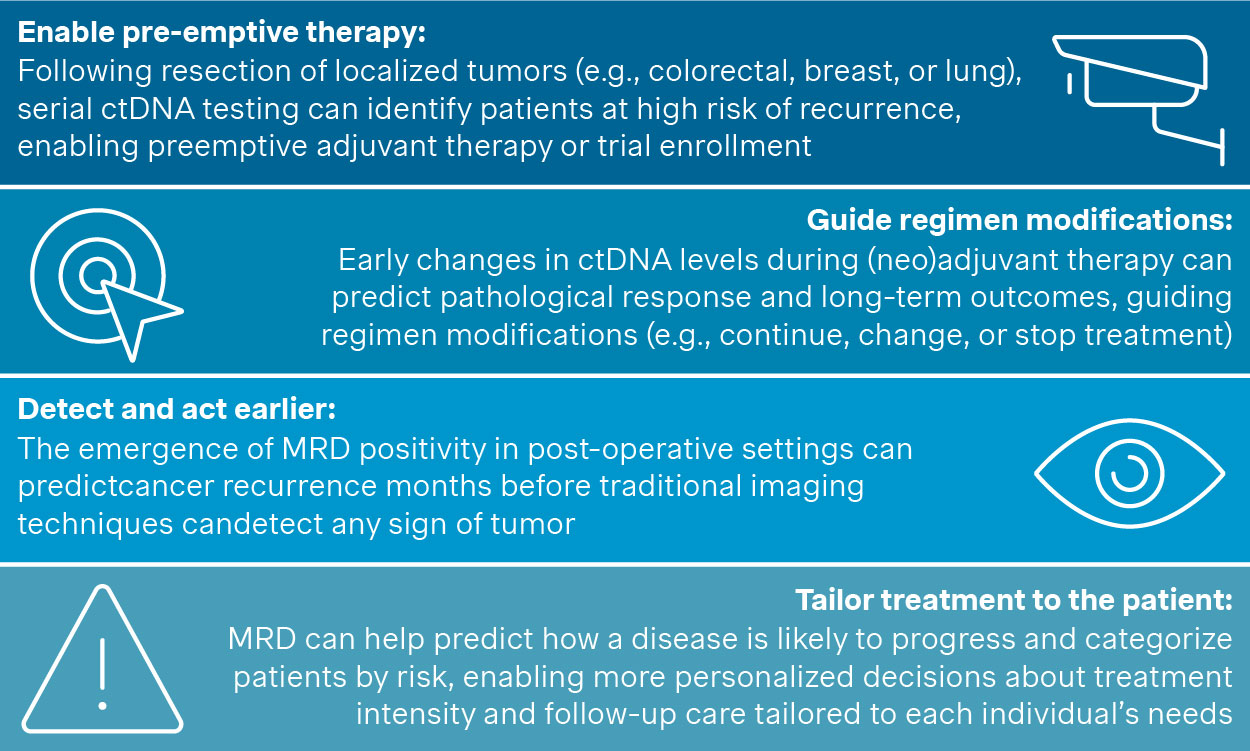
Figure 2. Clinical benefits of MRD in solid tumors
Potential economic benefits for MRD testing in solid tumors
In addition to direct clinical and health benefits, MRD’s impact can expand to economic value as well:
- Targeting adjuvant therapy to MRD-positive patients may reduce overtreatment and related costs
- Early recurrence detection enables less intensive salvage treatments, and therefore, can potentially reduce long-term health care expenses
Diagnostics and pharma manufacturers are now increasingly focusing on validating these potential clinical and economic advantages to drive MRD adoption forward. For example, cost-effectiveness analyses (e.g., from the DYNAMIC trial) and ongoing observational registries in e.g., breast cancer, are underway to collect budget impact data to inform payer perspectives.
Global market landscape for MRD innovations
Globally, the MRD market is evolving (Figure 3); however, the regional level of maturity differs.
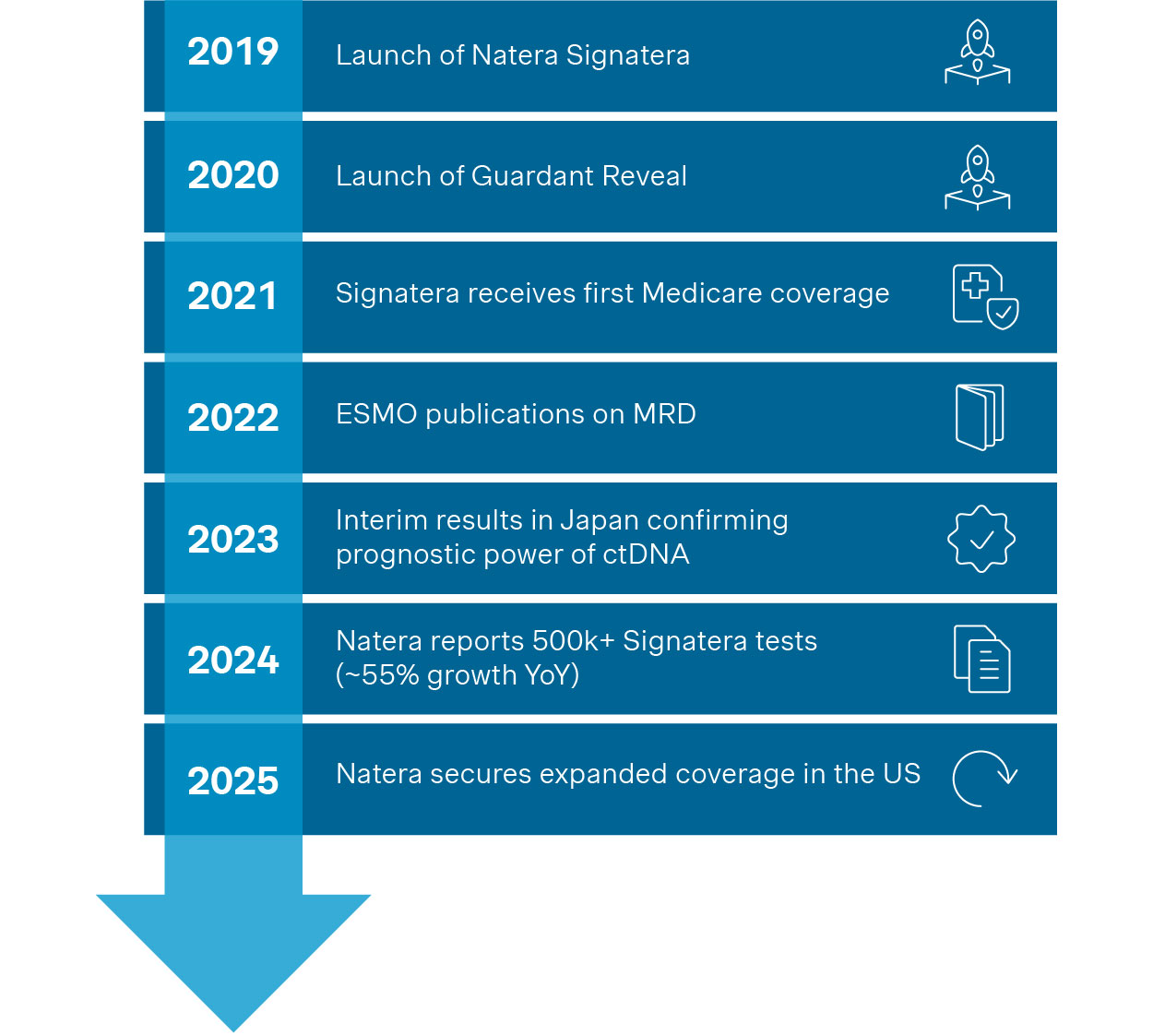
Figure 3. Key international milestones for MRD in solid tumors
MRD in the United States
The U.S. leads MRD adoption in solid tumors, driven by commercial ctDNA assays like Natera's Signatera and Guardant Health's Reveal. In 2024, Natera reported over 500,000 oncology tests performed (~55% YoY growth). Medicare now reimburses MRD testing across multiple cancers, including colorectal, breast, lung, bladder, and ovarian.
The NCCN recognizes ctDNA as a post-surgery high-risk marker in colorectal cancer and for surveillance in Merkel cell carcinoma. Though not yet universal, real-world use is growing: in the BESPOKE CRC registry, MRD results altered treatment plans in 16% of cases.
MRD in other markets
Interest and momentum outside the U.S. are growing, though progress remains uneven across markets:
- Europe: ESMO acknowledges ctDNA's potential, but guidelines remain cautious pending more robust evidence
- Japan: The CIRCULATE-Japan trial, the largest MRD study (>2,000 colorectal cancer patients) to date, confirmed ctDNA as a strong prognostic factor
- Australia: ): The DYNAMIC trial demonstrated that MRD-guided treatment can reduce chemotherapy use in stage II colon cancer without compromising Recurrence Free Surveillance
- China: Growing interest, including expert consensus on ctDNA-MRD in colorectal and lung cancers, though access and reimbursement remain limited
Currently, discussions around reimbursement and regulatory approvals are at an early stage, limiting widespread use beyond academic and research centers.
Pivotal ongoing trials laying the groundwork for MRD adoption globally
Meanwhile, a series of high-profile trials is building the evidence base that will shape how MRD is integrated into routine clinical practice globally. Some key examples include:
- COBRA / NRG-GI005 (USA, Colon Cancer): Phase III trial testing ctDNA-guided adjuvant chemotherapy in stage IIA colon cancer; results expected to influence U.S. guidelines
- MERMAID-1 (Global, NSCLC): Investigating ctDNA-based treatment adjustments post-surgery
- IMvigor011 (Global, Bladder Cancer): Following IMvigor010, this trial enrolls only MRD-positive patients post-cystectomy to test adjuvant immunotherapy benefits, building on subgroup analysis that revealed targeted benefit in MRD-positive cases
Recent developments
As of August 2025, MRD's competitive landscape has continued to evolve rapidly, with several noteworthy advancements reinforcing its momentum:
- Natera has secured broad Medicare coverage for its Signatera genome-based MRD assay and is set to expand its offering with a new tissue-free capability
- Personalis presented new ASCO data supporting the use of ctDNA MRD testing in cervical cancer
- Saga Diagnostics has entered the MRD space with a tumor-informed assay for breast cancer
- Qiagen and Foresight Diagnostics have announced a partnership to co-develop an NGS-based MRD companion diagnostic kit for lymphomas and other malignancies
- Quest Diagnostics received FDA Breakthrough Device Designation for its Haystack MRD test in stage II colorectal cancer
Collectively, these trials and other recent developments are setting the pace for evidence generation that can unlock regulatory alignment, guideline inclusion, and broader adoption. As outcomes mature, they are expected to catalyze MRD’s shift from a promising innovation to a foundational tool in solid tumor management.
Critical barriers to adoption for MRD in solid tumors
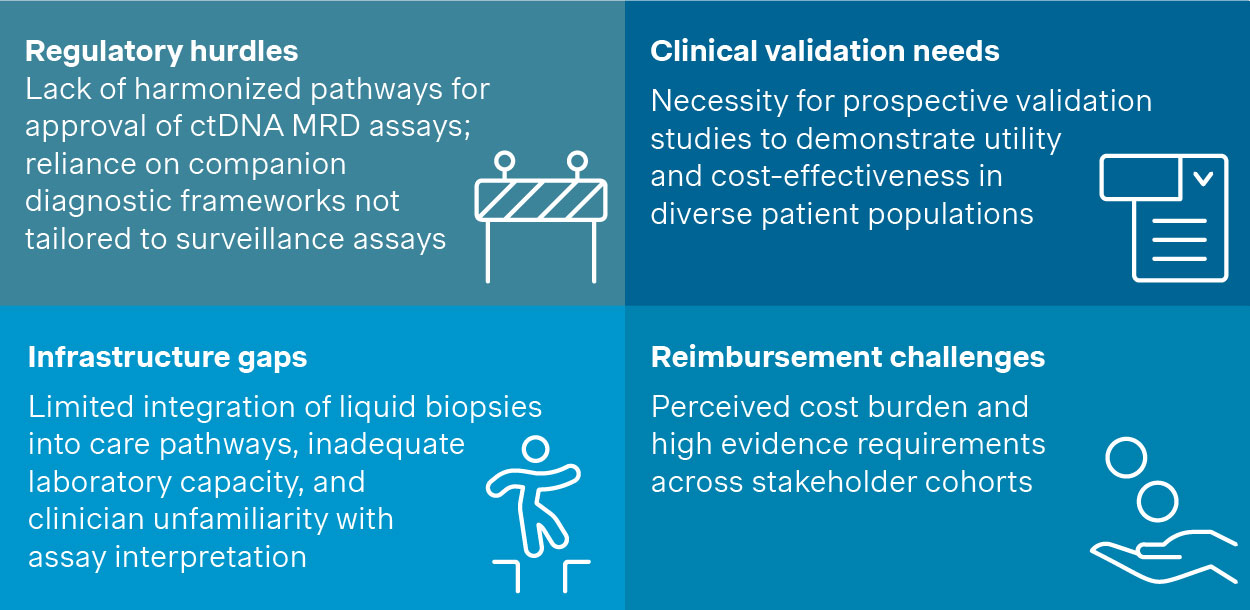
Figure 4. Critical barriers to adoption
The challenges outlined in Figure 4 are significant for any player looking to commercialize innovative MRD tests. Nevertheless, these barriers can be eased and/or overcame by establishing clear strategies.
The road ahead: Overcoming barriers for successful commercialization
While clinical validation and industry enthusiasm for MRD are growing, translating that momentum into sustainable commercial success requires deliberate, cross-functional action. Key stakeholders including diagnostic developers, biopharma partners, and healthcare systems must align on strategies that reduce uncertainty, demonstrate value, and build lasting market readiness. Four priority areas are emerging as essential for MRD assay manufacturers to facilitate this transition:
1. Evidence generation and regulatory strategy
- Launch global, harmonized trials with standardized endpoints
- Build longitudinal data registries tracking MRD status and outcomes
2. Payer engagement
- Develop market-specific cost-effectiveness models
- Pursue outcome-based reimbursement agreements early
3. Clinician and patient engagement
- Secure inclusion in major guidelines (e.g., ASCO, ESMO)
- Offer decision support tools integrating MRD into care pathways
4. Go-to-market execution
- Define clear value propositions aligned with payer needs / use cases
- Support sales teams with local data and value-based messaging
A transformative inflection point
The translation of MRD from hematologic malignancies to solid tumors marks a critical step forward in personalized oncology. By enabling ultra-sensitive detection of recurrence, MRD testing is poised to shape treatment decisions, improve patient outcomes, and generate economic value for healthcare systems. Its growing relevance makes it a priority for diagnostics and pharma players invested in the future of cancer care.
With deep expertise in diagnostics and precision medicine, we support clients across the product lifecycle - from early development to post-launch execution. We understand the nuances of MRD commercialization and deliver tailored strategies grounded in evidence, market insight, and executional rigor.
A special thanks to Giacomo Villa and Edoardo Tamborrino for their valuable contributions to the article.




