The biotech industry is full of organizations that are brilliant at science. However, many stumble once they need to turn that science into a business. Teams get swept up in the breakthrough, trial results, and mechanism of action, but forget that none of it matters unless patients get access and prescribers adopt.
It’s easy to get blindsided by a “loving science, being lost in science” mindset. However, great data doesn’t automatically translate into market success. The bar for differentiation in crowded therapeutic areas is incredibly high. By the time commercial and access teams get to the table, key decisions are already locked in: trial endpoints that aren’t payer-relevant, comparator arms that don’t reflect real-world practice, or data packages that miss what clinicians want to see.
This article explores why that gap persists, what it takes to close it, and how companies can move from being brilliant in the lab to being excellent at launch.
Commercializing drugs isn’t easy for first-time launchers. Why bother?
The odds are stacked against first-time launchers, with so many moving parts to get right: regulatory hurdles, market access negotiations, building awareness with physicians, and even patient support infrastructure. There’s a strong temptation to just license the product out to a bigger pharma that’s been through the grind before.
Experienced launchers have the muscle memory: they know how to activate their field forces, have payer relationships in place, and have already built patient services that can be tweaked rather than created from scratch.
First-time launchers can underperform by half or more compared to seasoned players in the early years, even when the science is equally strong. Not because the drug is less effective but because launch execution isn’t at the same level. That gap compounds: stumble in year one and payers remember, prescribers form habits with competitors, and your brand ends up permanently behind.
First-time launchers generate only a fraction of the revenue of experienced companies in the first four years post-approval
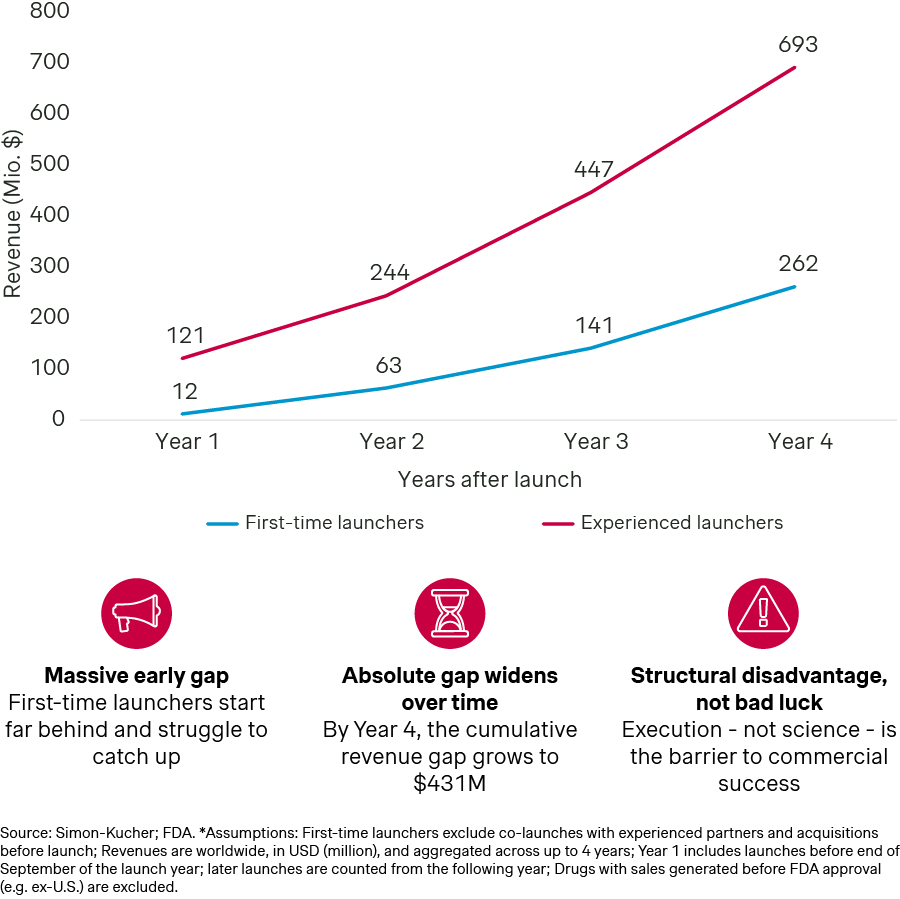
However, if you can pull off a launch yourself, you build credibility in the market, create the foundation for future launches, and show investors and partners that you’re more than a discovery shop. It’s also about control. When you commercialize yourself, you decide the positioning, the patient experience, and the long-term brand equity. If you give it away too soon, someone else decides what your innovation becomes.
Of course, the trade-off is risk. The burn rate goes up, timelines stretch, and mistakes can sink a company. But for some, the upside of proving you can go from bench to bedside under your own steam is worth it.
The first year on the market sets a product’s fate
In most cases, the first 12 months aren’t just relevant for key markets. Negative momentum can ripple into other market dynamics because regulators, payers, and KOLs watch each other closely. This is especially for biotechs, who live or die on the ability to show a positive trajectory quickly, fueling the next raise or positioning the company for acquisition. If revenue curves flatten too early, confidence drops, and the company’s strategic options narrow.
Widespread launch challenges
Unrealistic expectations for launch brands/ limited, wrong understanding of the opportunity
Inappropriate investment levels and resourcing
Divergent objectives and undefined, generic success factors
Limited alignment, visibility and quality of launch preparation
Year one is really the make-or-break moment. Once physicians get accustomed to prescribing patterns, or once payers anchor on a certain value narrative, it’s incredibly hard to shift them. Pharma teams should not underestimate the early start time for implementation. Excellence has to be built into the DNA of the organization well before approval.
Launch excellence: Not a single heroic push, but the overlap of three ongoing disciplines
Organizational alignment, organizational visibility, and execution quality with timeliness – launch success is about all three working together in a dynamic balance. You can have great alignment but no visibility, and the launch still struggles. Or you can execute like clockwork but without functional alignment, and the market narrative collapses.
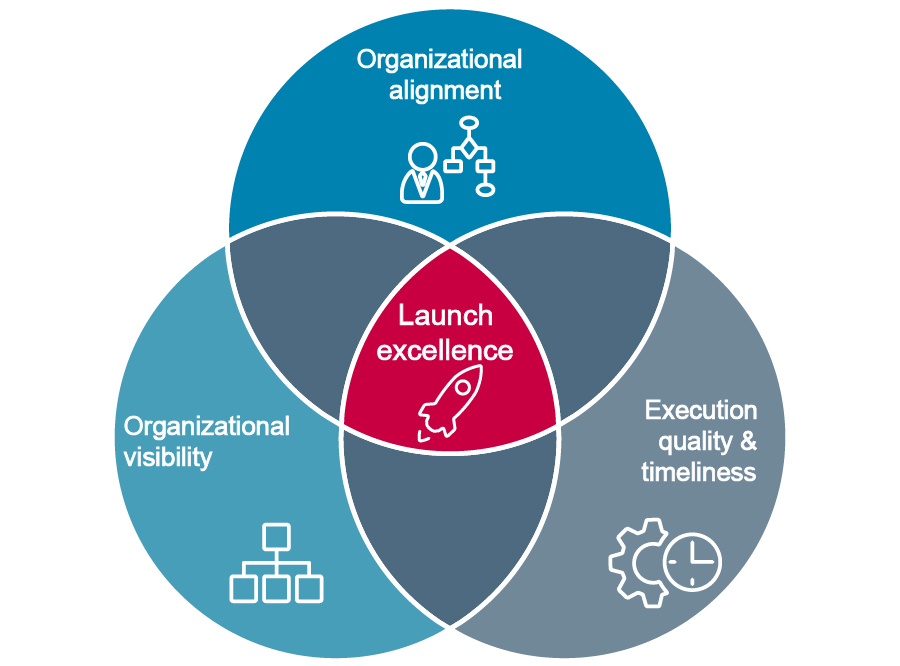
Organizational alignment
Biotechs that succeed in launch make alignment a top priority. They ensure that strategy execution is clearly understood at every level of the organization, from senior leadership to local teams in the field. This means breaking down silos between hierarchies and fostering genuine collaboration across functions. Medical, commercial, and access voices are brought together early and consistently, so that decisions are shaped with the full market perspective in mind.
Organizational visibility
Excellence also requires transparency. The best launch organizations provide clear visibility across functions and geographies, eliminating blind spots that can derail execution. Success factors and KPIs are defined and tracked well before launch, so progress can be measured against meaningful milestones. Importantly, local management is given the same real-time view of readiness as global leaders, ensuring that no market falls behind unnoticed. This visibility empowers teams to anticipate challenges and act before they become roadblocks.
Execution quality & timeliness
High-performing biotechs treat execution as a discipline in its own right. They manage the vast volume of launch activities with robust project management, ensuring nothing slips through the cracks. Interdependencies are mapped and monitored, so that every function understands how their work connects to others. Critical tasks are initiated early, avoiding the last-minute rush that undermines quality. And launches are resourced at the right level, balancing ambition with realism.
Successful launches aren’t one-off events. They’re a repeatable discipline built on the right people, processes, and technology. Program management expertise and cross-functional talent provide the structure to manage complexity and avoid silos. A strong technology backbone, ideally AI-enabled, automates routine tasks and delivers real-time visibility, allowing teams to focus on strategy, risk, and stakeholder engagement. The result is greater efficiency, accuracy, and alignment across the organization, leading to lower costs and stronger performance in the critical first year. More importantly, this infrastructure becomes the operating system for launch excellence, enabling companies to scale and replicate success across multiple products.
Key steps to successfully build a blockbuster
Launch excellence builds upon four key pillars which an organization needs to master individually for each target market and drug/technology launch.
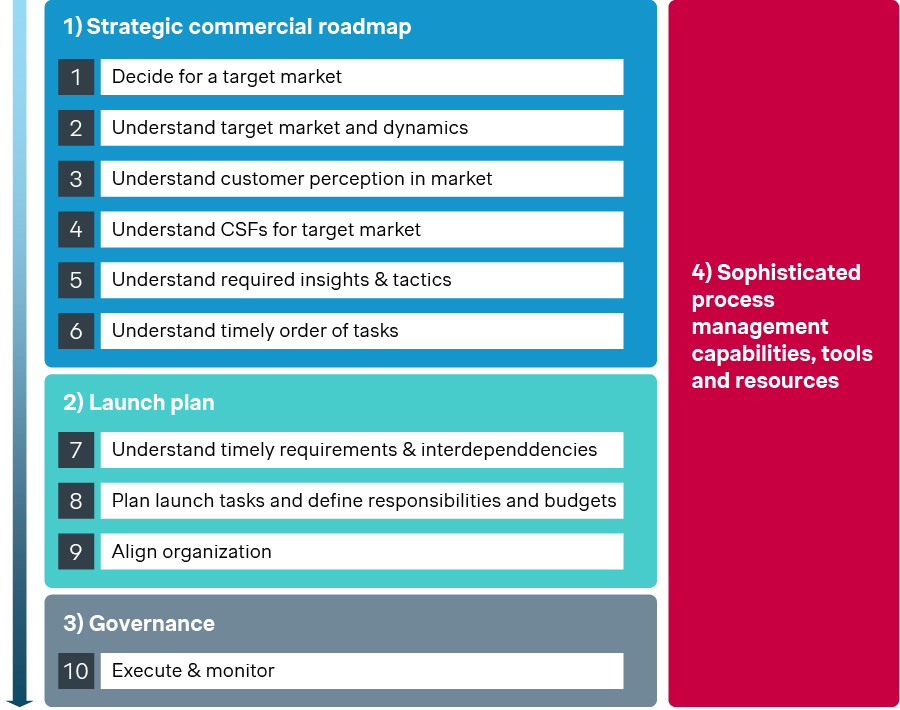
Strategic commercial roadmap: Define the market early, understand dynamics, and truly grasp customer perception. That’s how you avoid the trap of inflated expectations or clinical trial designs that don’t meet payer or prescriber needs.
Launch plan: Map interdependencies, set clear responsibilities, and align the organization. This addresses the huge complexity of launch tasks, avoiding late starts, lack of resources, and siloed execution, all which can be mitigated with discipline and foresight.
Governance: Sustain that alignment and visibility over time. Launches are dynamic; without governance structures to monitor, escalate, and adjust, you end up with static plans that quickly become irrelevant. Governance is what ensures risks are spotted and acted on early.
Sophisticated process management, tools, and resources: Launch excellence isn’t just about smart people and strategy but about building the infrastructure. Dashboards, PMOs, risk tracking systems, etc. allow large, cross-functional teams to operate in sync over months and years.
Planning is not enough for a successful launch
Launch excellence is a continuous cycle. It’s crucial to set clear expectations, measure progress, make ownership explicit, and adjust quickly when needed.
KPIs anchor ambitions in measurable outcomes tied to critical success factors (CSFs). They also enable early detection of when something is off-track, which is that “insurance policy” we discussed.
Clear targets avoid inflated projections and create achievable, motivating goals. Misaligned expectations are one of the root causes of underperformance.
Tracking (who, what) creates organizational visibility. Launches fall apart when functions work in silos, or when leadership doesn’t have real-time readiness insight. Linking tracking to a RACI matrix ensures accountability: everyone knows who owns what, and everyone sees the same reality.
Course correcting closes the loop. Launches are dynamic, and without agreed “what if” scenarios, teams often react too late or inconsistently. Embedding structured course correction ensures agility. Static plans are almost guaranteed to become outdated.
Launch excellence is about much more than good science. It’s about building the alignment, visibility, and execution muscle to ensure that great therapies reach patients and achieve their commercial potential. For biotechs, the stakes are high, but so is the opportunity.
This is where Simon-Kucher can help. We’ve guided countless life sciences companies through the journey from breakthrough science to successful launch, combining deep industry expertise with proven frameworks for launch readiness, governance, and commercial excellence.




