Diagnostics barriers keep many patients from benefiting from targeted therapies. Discover six key watchouts and how pharma can improve test access and unlock the full value of precision medicine.
Precision medicine is a top priority for many pharmaceutical companies, driven by its potential to deliver highly targeted therapies that improve patient outcomes. Data shows that it now accounts for over one-third of FDA drug approvals, with the number of new FDA-approved biomarkers doubling every five years. Commercial success, however, depends on more than the ability to identify biomarker-positive patient population. It requires guiding them through the diagnostic journey, which involves four key steps:
- Awareness of biomarker test timing and purpose: Physicians understand the need for biomarker testing and how the targeted therapy is a potential treatment option.
- Test ordering: Physicians obtain the clinical sample materials from the patients and order the test from a qualified laboratory.
- Test execution: The laboratory conducts the biomarker test and assesses the outcome.
- Treatment decision: The laboratory provides the results to the physicians who make an informed treatment decision.
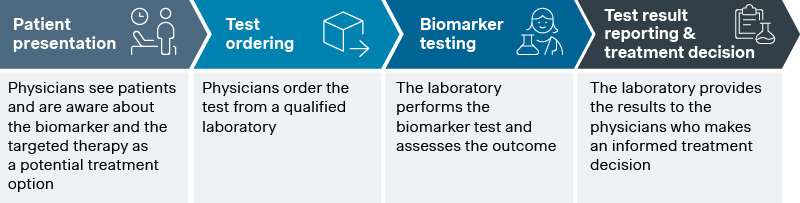
Source: Simon-Kucher insights
Critical pitfalls that disrupt the diagnostics pathway
Barriers can arise at every stage, preventing patients from receiving appropriate treatment. To address these challenges, pharma companies need to monitor seven critical areas along the diagnostic journey:
- Physician understanding of biomarkers and their clinical utility
One of the primary challenges is that many physicians are unaware of the clinical utility of novel biomarkers. For example, multiple guidelines recommend molecular testing in non-small cell lung cancer (NSCLC) before first-line therapy. Despite this, more than half of all patients do not receive molecular biomarker testing at the start of the therapy, indicating the need to strengthen awareness and belief in its value.
- Availability of clinical sample materials
Sample material shortages can occur when multiple biomarker tests are already established in an indication, or when biopsies are highly invasive and physicians do not want to put their patients at risk. In NSCLC, 100 µm total tumor tissue material is required and a study found that around 15% of patients were unable to receive biomarker testing due to insufficient tissue.
- Clinical positioning of the biomarker test
Pharma companies aim to ensure that testing for their associated therapy is prioritized above other biomarkers. However, biomarkers are often only tested at specific lines of therapy. This means that many eligible patients at later lines never get tested as their health deteriorates, they become ineligible, or they die before testing occurs.
- Laboratory capacity and capability to perform biomarker test
Capacity constraints in laboratories can limit the availability of testing options. For example, as diagnostic companies promote their next-gen platforms and shift menu expansion investments toward newer biomarker tests, there is a significant risk of delays in platform adoption, market penetration, and test availability across markets.
- Effort for workflow integration of new biomarkers
New biomarker test introductions, such as TROP2 quantitative continuous scoring (QCS) on computational pathology platforms, require changes to established workflows, internal validation, and staff training. These requirements can create bottlenecks as laboratories are careful not to disrupt their current operations.
- Quality of test delivery
Variation in test quality across different laboratories can affect the accuracy and reliability of results. Inconsistent test results may lead to misdiagnosis or inappropriate treatment plans, undermining the trust in precision medicine.
- Conversion rate of test results to treatment decisions
Delays in test turnaround times can prevent patients from accessing the right treatment options promptly. For instance, a study found that it can take over three weeks from test order to receiving results for patients with colorectal cancer (CRC).
Best practices for pharma to improve diagnostics access and adoption
As pharma companies closely monitor these barriers, targeted action often becomes necessary. The following best practices can help improve availability and adoption of companion diagnostics for associated targeted therapies:
- Create dedicated education materials for biomarkers and companion diagnostics
Webinars, workshops, and detailed guides help educate physicians about the clinical utility and benefits of novel biomarkers. This can increase awareness and encourage the adoption of these tests in clinical practice. For example, AstraZeneca has created a Biomarker Testing Video library to educate physicians in the US.
- Actively support stewardship programs to improve clinical sample material availability
Collaborating with healthcare providers to promote best practices in sample collection and handling is key to ensuring efficient collection and use of clinical samples. This was clear when Roche sponsored a webinar which discussed optimal tissue sample handling to increase actionable tumor information. BMS took it one step further by supporting ctDNA assay development and dual tissue-blood testing to overcome biopsy limitations for TMB NGS testing.
- Advocate for reflex testing protocols to remove risk of physicians not ordering the test
Reflex testing ensures that biomarker tests are automatically ordered based on predefined clinical criteria to ensure patients receive the necessary treatment. For example, Amgen funded a study that highlighted how molecular reflex testing improves turn-around-time of test results, enabling physicians to make targeted treatment decisions faster.
- Develop hub and spoke networks to streamline the diagnostic journey and avoid delays in diagnosis and treatment
Testing networks can expand access to novel biomarkers or new testing technologies, particularly in markets with a maturing diagnostic landscape. Examples include AstraZeneca’s AZFastNet network in Italy to support BRCA1/BRCA2 and EGFR testing or Bayer’s collaboration with NeoGenomics to provide Test4TRK Program at no cost for NTRK NGS testing.
- Sponsor EQA programs to support standardization of testing protocols and delivery across laboratories
Participation in EQA programs help laboratories establish standardized testing protocols and improve consistency in test delivery. For instance, Astellas sponsored a global ring study in 2023 to support the validation of commercial Claudin-18 antibodies in gastric cancer testing.
- Provide resources for efficient test turnaround times and actionable reporting to enable timely treatment decisions
Offering comprehensive training programs and educational resources can significantly improve test turnaround times and support more confident treatment decisions. Consider how BMS and Merck sponsor various microlearning activities for pathologists around PD-L1 testing. Agilent also supports this effort as a diagnostic partner, offering a global Biomarker Pathologist Training Program that helps pathologists score biomarkers accurately and confidently.
Helping you drive diagnostics success in precision medicine
These dynamics show the need for pharma companies to take a more active role in shaping the diagnostics ecosystem to support their precision medicine assets. To do this effectively, companies must thoroughly assess the landscape and develop a commercial diagnostics strategy to obtain a clear roadmap with prioritized initiatives.
This requires detailing and planning to develop key materials, tools, and country-level rollout plans, ensuring local teams are equipped and engaged to collect data and generate evidence and align on roles and responsibilities for prioritized initiatives with the diagnostic partner. Finally, action plans and initiatives need to be executed and monitored post-launch by local teams to ensure successful implementation and coordinated engagement with ordering physicians and labs.
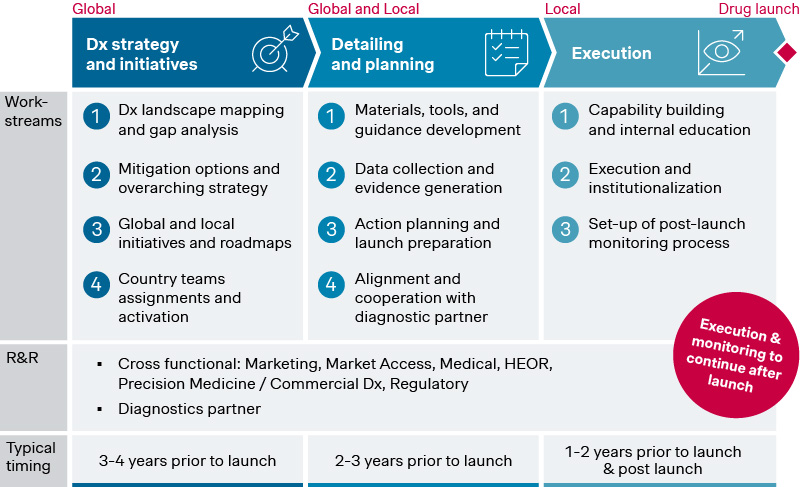
Source: Simon-Kucher insights
As your trusted partner with extensive subject-matter expertise and experience across precision medicine and diagnostics, Simon-Kucher can support you through each of these steps. If that has piqued your interest, feel free to reach out and we will be happy to continue the conversation!