Immunization is a core component of public health policy and represents a low-cost preventive option against diseases with enormous health and cost burden. Our experts discuss how recent US policy changes, most notably the Inflation Reduction Act (IRA), have expanded patient access to immunization.
In recent years, major policies enacted by the US government have promoted immunization access. Measures include broadening vaccine coverage by government payers and bolstering vaccine administration at the pharmacy level. These policies have impacted multiple pillars of the US healthcare system, including:
- Medicare: The IRA broadened vaccine coverage without patient copay to include all ACIP-recommended routine and non-routine vaccines. This includes those falling under Part D, eliminating any cost sharing for vaccine access for Medicare Part D and Medicare Advantage patients. This requirement came into effect in January 2023.
- Medicaid: Similarly, the IRA mandated that State and Managed Medicaid plans cover all ACIP-recommended vaccines for adult patients with no copay, aligning coverage across Medicaid populations. This mandate took effect in October 2023.
- Pharmacies: The COVID-19 pandemic accelerated a US trend of vaccine administration at retail pharmacies. The extension of the Public Readiness and Emergency Preparedness (PREP) Act of 2020 underlines the importance of US pharmacists as vaccine administrators.
Recent US policies have promoted broad vaccine access across commercial and government healthcare plans
The Vaccine For Children (VFC) program has been in place since 1993 to ensure vaccine access for children under the age of 19 who are Medicaid-eligible, uninsured, American Indian, or Alaska Native. Then, in 2010, the Affordable Care Act (ACA) represented a major regulatory overhaul and expansion of healthcare in the US, including expanded access for adult vaccines.
Specifically, the ACA mandated that employer-sponsored commercial healthcare plans cover all immunizations recommended by the CDC’s Advisory Committee on Immunization Practices (ACIP) and listed on the CDC’s adult immunization schedule (i.e., routine immunizations for influenza, MMR, and others) without patient cost sharing.
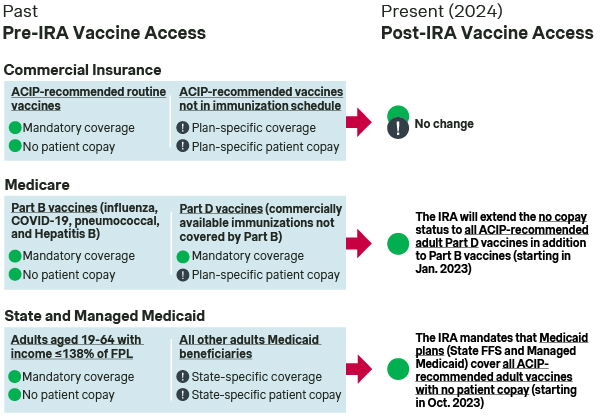
Though the ACA broadened immunization coverage by commercial health insurers, Medicare and Medicaid historically provided less consistent vaccine coverage for adults in both the breadth of immunizations covered and the patient copay for access.
- Medicare Part B: Specific vaccines (i.e., influenza, COVID-19, pneumococcal, and Hepatitis B) are designated by CMS as being covered under Medicare Part B with no patient copay – notably distinct from the 20% patient cost sharing for most other Part B covered drugs and services.
- Medicare Part D: CMS previously required Part D plan sponsors to cover all commercially available vaccines not covered under Part B. Part D plan sponsors had flexibility in determining patient copay levels. As a result, patient cost sharing for vaccines varied significantly across different immunizations and plans.
- Medicaid: The ACA mandated Medicaid coverage of all ACIP-recommended routine immunizations with no patient copay for adults aged 19-64 with an income at or below 138% of the federal poverty level. However, Medicaid beneficiaries outside of the ACA mandate faced state-to-state variability in adult vaccine access and cost-sharing, due to these limited requirements for Medicaid adult vaccine coverage.
The Inflation Reduction Act (IRA) was signed into law in August 2022 and had broad implications for the US Healthcare system, including expanding vaccine access for adult Medicare and Medicaid beneficiaries. Meanwhile, state-specific policies have generally enhanced the role of pharmacies in vaccine administration, thereby facilitating patient access to sites of immunization care.
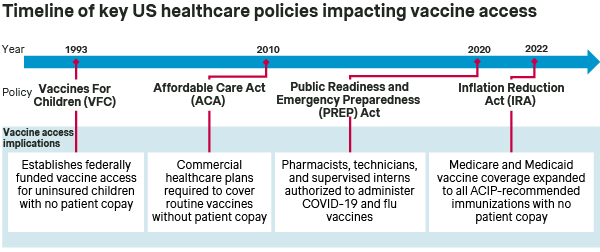
BETTER ACCESS: The ACA and IRA significantly expanded vaccine access in the US by broadening coverage - specifically to encompass ACIP-recommended immunizations - as well as eliminating patient copays. To this end, healthcare providers and vaccine manufacturers are assured that immunizations recommended by ACIP will be covered by commercial, Medicare, and Medicaid plans, and that patients will not face financial hurdles. At the same time, the continued expansion of pharmacies as sites of vaccine administration further promotes patient access to immunizations.
The IRA removed inconsistencies in Medicare vaccine coverage by mandating coverage of ACIP-recommended Medicare Part D vaccines with no patient copay
Despite coverage of influenza, pneumococcal, Hepatitis B, and COVID-19 vaccines through Medicare Part B with no copay, coverage of all other vaccines through Part D vaccines has historically been variable between plans. Part D vaccines were subject to payer decisions on coverage, formulary tier placement, and patient cost sharing.
A 2011 report from the GAO found that Part D beneficiary out-of-pocket costs for vaccines and administration in 2009 ranged from $0-$195 for a shingles vaccine and $0-$70 for a tetanus vaccine. In addition, the report found that more than 80% of physicians cited beneficiaries’ lack of insurance coverage (83%) or beneficiaries’ difficulty affording the cost sharing for the vaccine (85%) as reasons for not stocking shingles vaccines. The variability in Part D vaccine coverage and out-of-pocket costs were hurdles to vaccine access.
The IRA mandate for Medicare Part D coverage of vaccines went into effect in January 2023. Ahead of the change, CMS issued guidance for Medicare Part D and Medicare Advantage plan sponsors:
- Coverage: CMS specified that the IRA coverage mandate applied to “all categories of ACIP recommendations”, including recommendations based on shared clinical decision-making, for use in limited populations, or those not included on the CDC/ACIP Adult Immunization Schedule for routine immunization.
- Cost sharing: CMS specified that there should be no cost sharing for beneficiaries for vaccine ingredient costs, sales tax, dispensing fees, or administration fees.
The CMS guidance wording makes the mandated IRA vaccine coverage broader than the mandated ACA commercial insurance vaccine coverage, as the latter is limited to routine vaccines. The IRA impact is already visible in healthcare provider materials from GSK on Shingrix, which shifted from communicating in 2021 that the majority of Part D patients “pay an out-of-pocket cost that is less than $50 per dose” to being able to “receive SHINGRIX for $0 at the pharmacy” in 2023. The IRA mandate harmonized vaccine coverage across Medicare Part B and Part D at no cost to beneficiaries, simplifying vaccine coverage and removing potential access barriers for Medicare beneficiaries.
BETTER ACCESS: The IRA mandated coverage of all ACIP-recommended immunizations under Medicare Part D with no patient copay. This change has removed the imbalance in coverage between historically designated Part B and Part D vaccines to ensure consistent access for Medicare beneficiaries regardless of their Medicare plan or vaccine.
The IRA mandated that Medicaid cover all ACIP-recommended vaccines with no patient copay for all adult beneficiaries
Adult vaccine coverage by Medicaid has historically varied drastically across states, where states inconsistently covered ACIP-recommended vaccines prior to the IRA. The IRA mandate sought to address disparities in Medicaid vaccine coverage.
In June 2023, the Centers for Medicare & Medicaid Services (CMS) released guidance to clarify the IRA mandate for State and Managed Medicaid, particularly with regards to the breadth of vaccine coverage and patient cost sharing:
- Coverage: The CMS guidance mirrored that for Medicare and specified that the IRA mandated coverage of all categories of ACIP-recommendations. Covered categories expanded beyond routine immunizations, and included ACIP-recommended vaccines that are not on the immunization schedule or recommended based on “health condition, occupation, and travel”.
- Cost sharing: The guidance clarifies that vaccines and their administration must be covered by Medicaid without patient cost sharing for both fee-for-service (FFS) and managed care Medicaid plans.
The IRA mandate for Medicaid vaccine coverage came into effect in October 2023, and therefore remains a developing trend as states continue to roll-out coverage changes and communications.
Thus far, states have exhibited distinct approaches to this implementation. For example, the Texas Medicaid & Healthcare Partnership (a State Medicaid contractor) proactively published a guidance for managed care organizations. This guidance clarified that vaccines would be covered for Medicaid beneficiaries without patient cost sharing and specified billing codes to facilitate implementation. Conversely, other states with large Medicaid populations (e.g., Florida) have not provided clarity or communication to beneficiaries on vaccine coverage.
Overall, while the IRA and CMS guidance are clear in mandating vaccine coverage of ACIP-recommended vaccines at no patient cost, states have taken inconsistent approaches to communicating the broadened vaccine access to Medicaid beneficiaries.
BETTER ACCESS: The IRA has mandated Medicaid coverage of a broad range of ACIP-recommended adult immunizations with no patient copay. While this will enable broad and uniform Medicaid vaccine access across the US, the implementation of this mandate is ongoing as State Medicaid agencies differ in their roll-out and communication approaches.
Recent COVID-19 pandemic policies expanded access to vaccines at pharmacies
In recent years, retail pharmacies have taken on a central role in vaccine administration. Their operating model presents several advantages that together improve access for patients. These advantages include longer business hours than many PCPs, no need for a patient-provider relationship, and visibility and proximity of community pharmacies.
Currently, pharmacists may administer vaccines in every state, DC, and Puerto Rico, but there is substantial state-to-state variability around eligibility of patients under 18 years old and the requirement for a physician prescription. Unlike states that gave broad prescribing and administration authority to pharmacists for ACIP-recommended vaccines (17 states according to NASPA), Missouri and North Carolina still require physician prescriptions prior to pharmacist administration for certain vaccines or circumstances.
The COVID-19 pandemic brought significant attention to state-to-state gaps in vaccine access and precipitated legislation to uniformly expand the role of pharmacies in both emergency and routine immunization. The amended PREP Act of 2020 authorized pharmacists, pharmacy technicians, and supervised pharmacy interns country-wide to administer COVID-19 vaccines to ages 3+, influenza vaccines to ages 19+, and all routine childhood vaccines, superseding state-specific limitations at the time.
The PREP Act’s pharmacy immunization authorization was extended in April 2023 from the end of the national public health emergency (May 2023) to December 2024. Before this announcement, individual states had already introduced measures to extend the Act’s provisions on pharmacy practice scope. These measures expanded vaccine administration at pharmacies, leading to more than half of COVID-19 vaccine doses being administered in pharmacy settings by the end of the public health emergency.
Despite these trends, challenges remain that hinder the further adoption of pharmacy-based immunization, including:
- Risk of unfavorable reimbursement depending on patient insurance
- Limited pharmacist experience with non-routine immunizations
- Insufficient staff availability for vaccine administration
- Local stocking of only some high-demand vaccines
Though federal and state policies contributed to improving vaccine access at pharmacies, further policy improvements on these fronts could further expand access to vaccines in the US.
BETTER ACCESS: Pharmacies have gained importance as a site of vaccine administration, driven in part by the PREP Act of 2020, which aimed to remove state-to-state variability in ability of patients to receive vaccines at pharmacies. Future policies may still be needed to remove further hurdles faced by pharmacies with regards to vaccine administration.
Despite some remaining gaps in immunization coverage, future policies will likely continue the trend toward better vaccine access
Despite general trends toward broadened vaccine access across commercial and government US healthcare plans, there are some remaining gaps:
- Commercial: Notably, the ACA required that commercial healthcare plans cover ACIP-recommended routine immunizations with no patient copay, but this level of access is not required for elective immunizations. As a result, the commercial coverage and patient copay levels for ACIP-recommended non-routine immunizations (incl. vaccines for Dengue, Ebola, Yellow Fever, Chikungunya) is determined on a plan-specific basis.
- Medicare and Medicaid: While CMS guidance for Medicare and Medicaid have sought to clarify that the IRA mandates coverage without patient cost sharing for all ACIP-recommendation categories, there are remaining uncertainties around the coverage of specific vaccines (e.g., elective vaccines recommended by ACIP) that might require greater communication from state Medicaid agencies to overcome.
Future US policies may continue to promote better access to immunizations. Specifically, the Biden administration’s proposed 2025 budget includes funds for a “Vaccine for Adults” program – similar to the VFC – that would provide uninsured adults access to routine and outbreak immunizations at no cost. It will also be interesting to monitor any state or national policies on pharmacy vaccine administration that could arise once the extended PREP Act expires in December 2024. It will be important for healthcare providers and vaccine manufacturers to monitor these policy changes and ensure pull-through of these policies to unlock better vaccine access for patients.
BETTER ACCESS: While recent federal policies have promoted vaccine access in the US, there are still some remaining gaps and uncertainties in the breadth of coverage. Future policies could further facilitate access to vaccines for all patients regardless of insurance status and independent of whether the vaccine is administered at a pharmacy or in a physician's office.
Thanks to Sara Bi for contributing to this article!
Better Market Access
The role of better market access is to remove any hurdle that prevents or hinders patients from receiving available treatments. In the right place, and at the right time.
Today, the impact of market access spans clinical development, regional commercial activities, patient engagement, and post-launch compliance. Yet, for many pharmaceutical companies, planning for commercialization only truly begins when a drug has been submitted for approval — far too late in the process.
Get to know our insights on local trends, regional and global developments and global to local excellence.